Replacing Working Group Glass Ceilings with Enforceable Minimal Standards (clickable video chapters below and on YouTube site) Further to our recent statement regarding major UK announcements, the above presentation provides the solution to concerns about new DHSC ME/CFS Delivery Planning mechanisms and final implementation. Current glass ceilings will limit success of this new process and…Continue readingNormalised Medical Rule Breaking and the UK DHSC ME/CFS Review
Tag: Liability
Education, Collaboration and Policy Planning Initiative Following a generous private grant of resources targeted specifically at Project Florence, we are proud to publicly announce the initiative, which is now moving into a test phase following pandemic driven delays. Doctors with M.E. Registrants and Strategic Partners will benefit from a new digital platform that unifies education…Continue readingProject Florence Grant – Unified Digital Platform for Postviral Disease Professionals and Practitioners
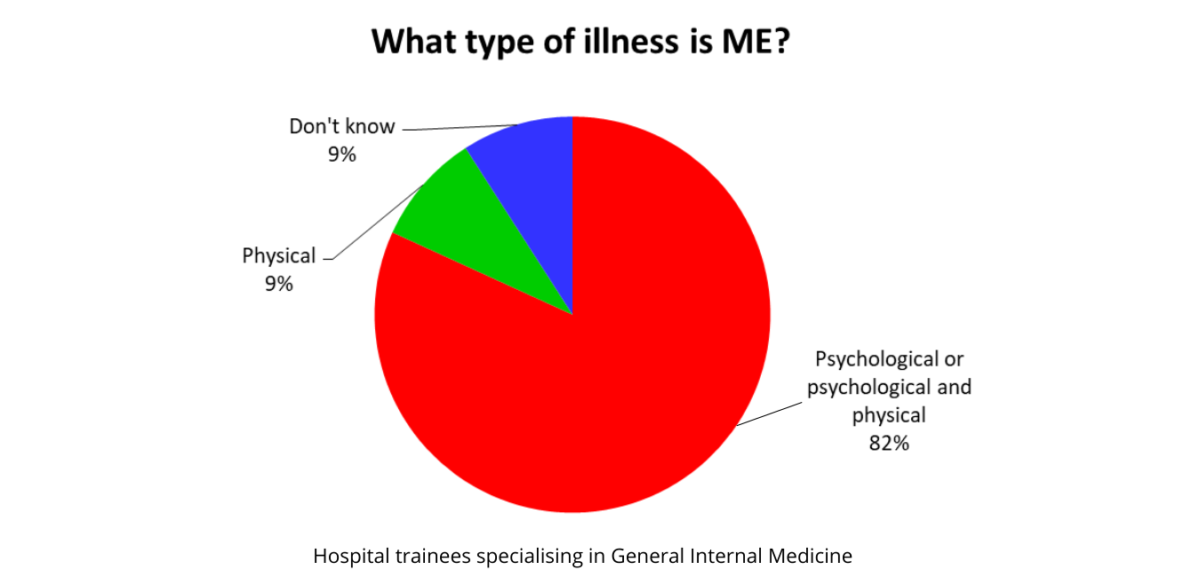
An Audit of UK Hospital Doctors’ Knowledge and Experience of Myalgic Encephalomyelitis Doctors with M.E. Founder and Director Dr. KN Hng, Director Dr. Keith Geraghty and Honorary Fellow Prof. Derek Pheby report their findings regarding ME/CFS knowledge and understanding amongst the medical community following an audit of hospital doctors at a training event in the…Continue readingPoor knowledge of ME/CFS among doctors puts patients at risk of harm
A call for change in the face of long-standing regulatory failure The following public communication from our Governance Board regards the opportunity for a new “Medical Regulatory Agency”, ready for the post-brexit and the machine learning digital eras. The letter also contains background pertaining to long-standing regulatory failure, unlawful medical norms, contra-scientific medical belief systems and…Continue readingPublic Letter to DHSC, MHRA, HoC, NICE: Call for a ‘Medical Regulatory Agency’
It is common that patients with ME/CFS must repeatedly pursue enforcement action of their pre-existing rights under statutes, policy and common law. It also known that successful enforcement of pre-existing rights can be followed by retributory abuse of power and authority. It is known that flagrantly retributory liability generation can include punishing qualified professionals who…Continue readingObligation to not abuse power and authority
Care should be taken to reassess existing expertise sources in the field of post-viral disease and to distinguish between 1) the marketability or familiarity of expertise versus 2) disproportionate risk of divergence from legally sustainable standards. It is rare for institutionalised medical norms to exclude scientific consensus to the degree witnessed in this field. This…Continue readingExpertise procurement risk and reputational risk – medical and legal
Although the jurisdictional focus is the United Kingdom, direct equivalence can be found in other jurisdictions and markets. The policy, contractual and legal requirements on medical practice and indemnification provision follow a similar structure in most locales: neither official edict, professional body, private contract, the habitual nature of unlawful care nor eminently misinformed obfuscation can…Continue readingJurisdictions and markets (UK and international)
The absolute clarity of the legal status of ME/CFS is of immediate risk management importance, due its statutory, policy and third-party contractual implications frequently being ignored. These stem from habitually unlawful clinical judgement and arbitrarily discriminative administrative treatment that fail to account for the clarity of classification and associated lawful obligations. The absolute clarity of…Continue readingObligation to not negate ME/CFS legal status
The law does not allow clinical judgement to be discriminatory or to breach duties of care – a normally uncontroversial statement with an unusual degree of unmanaged consequences for this field of disease. The law does not allow clinical judgement to be discriminatory or to breach duties of care – a normally uncontroversial statement with…Continue readingUnlawful clinical judgement (flu and covid vaccination examples)
Informed by the scientific, statutory and policy contexts outlined above, the following simple tests of clinical judgement lawfulness further outline lawful policy implementation requirements, mitigating the elevated probability of normalised unlawful clinical judgement in this context. Contrary conclusions constitute unlawful discriminatory implementation of policy, an arbitrary evasion of clear wording, thus discriminating on the very…Continue readingTests of clinical judgement lawfulness