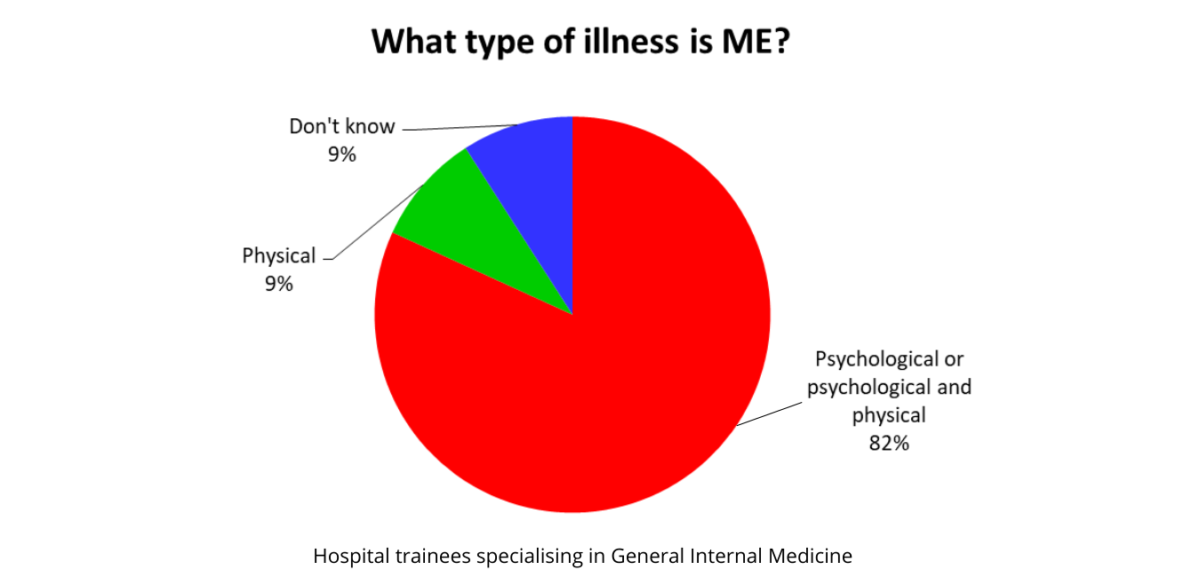
An Audit of UK Hospital Doctors’ Knowledge and Experience of Myalgic Encephalomyelitis Doctors with M.E. Founder and Director Dr. KN Hng, Director Dr. Keith Geraghty and Honorary Fellow Prof. Derek Pheby report their findings regarding ME/CFS knowledge and understanding amongst the medical community following an audit of hospital doctors at a training event in the…Continue readingPoor knowledge of ME/CFS among doctors puts patients at risk of harm
Tag: medical norm
Traducido por / Translated by Dr. Esther GarcésDoctors with M.E. Associate, Family Physician and RadiologistMédico especialista en Medicina Familiar y Comunitaria y Médico especialista en Radiodiagnóstico Contenido Descripción General La Encefalomielitis Miálgica (EM) —también conocida como Síndrome de Fatiga Crónica (SFC)— es una enfermedad orgánica crónica, compleja y multisistémica con consecuencias, a menudo, devastadoras. Se…Continue readingLa guía NICE EM/SFC aplicada a la práctica en atención primaria
Sumber asal diterbitkan dalam Bahasa Inggeris pada 15/1/2022. Diterjemahkan oleh / Translated byDr Hannah Nazri MBChB, BSc (Hons), MSc (Oxon)Doctors with M.E. Associate, clinician scientist, entrepreneur, and women’s rights & education advocate.Instagram | Twitter | LinkedIn | Blog Kandungan Gambaran keseluruhan Myalgic Encephalomyelitis (ME), juga dikenali sebagai Sindrom Kelesuan Kronik (Chronic Fatigue Syndrome – CFS),…Continue readingDari garis panduan kepada praktikal: Implikasi NICE ME/CFS buat doktor-doktor pakar perubatan keluarga (GP)
Publié initialement en anglais le 15/01/2022. Veuillez nous excuser, nous sommes conscients qu’il y a un problème de ponctuation. Nous faisons notre maximum pour le résoudre au plus vite. Traduit par Millions Missing France Contenu Vue d’ensemble L’encéphalomyélite myalgique (EM), également connue sous le nom de syndrome de fatigue chronique (SFC), est une maladie biologique…Continue readingMise en pratique : Ce que l’EM/SFC signifie pour les médecins généralistes, selon les préconisations du NICE
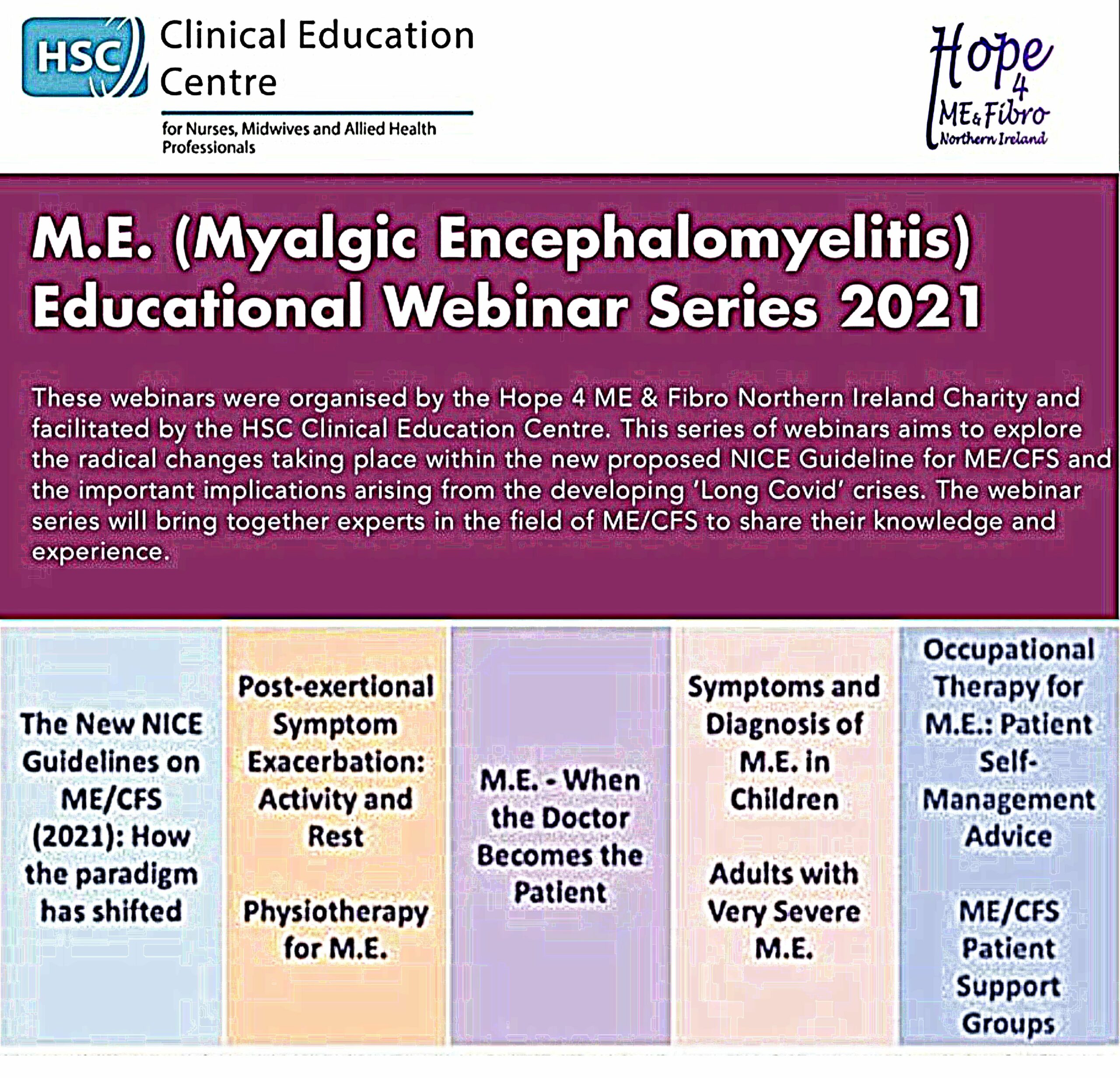
We are pleased to announce that the M.E. (Myalgic Encephalomyelitis) Educational Webinar Series organised by partner organisation Hope 4 ME & Fibro Northern Ireland and hosted by the Health and Social Care Clinical Education Centre of Northern Ireland is now available for public viewing. Featuring experts and speakers from Hope 4 ME & Fibro N.I.,…Continue readingMyalgic Encephalomyelitis: Northern Ireland Educational Webinar Series
Contents Overview Myalgic Encephalomyelitis (ME), also known as Chronic Fatigue Syndrome (CFS), is a chronic, complex, multi-system biological illness with often devastating consequences. It affects all age groups including children and all social classes. About 75% of sufferers are female. It has a worse quality of life score than many other serious illnesses including cancer,…Continue readingPutting it into Practice: What NICE ME/CFS means for GPs
Doctors with M.E. stands ready to work with partners and organisations to bring medical education up-to-date and assist in developing the right services for ME/CFS patients. “The new guideline represents a positive and total paradigm change, uniting around the science, official disease classification and medico-legal compliance implications.” “This translation of scientific knowledge into clinical practice…Continue readingNICE 2021: A Triumph of Science over Discrimination
The current NICE debacle and extraordinary deviation from normal procedure and published protocol is further evidence of institutionalised discrimination by large sections of the UK medical establishment. That one professional and patient group should be treated in a manner at odds with clear and growing scientific knowledge would be unacceptable in any other disease. Yet…Continue readingThe NICE debacle – will NICE survive?
Doctors with M.E. today formally announces two Honorary Fellows, recognised for the value of their contributions to ME/CFS scientific progress, Professors Ron Davis and Brian Hughes. Professor Ron Davis is a global authority on biochemistry, genetics and genomics, Director of the Stanford Genome Technology Center (SGTC) at Stanford University and Director of the Scientific Advisory…Continue readingFounding father of Human Genome Project joins Doctors with M.E. with leading evidence based policy expert as Honorary Fellows
Science’s rejection of psychological dogma Doctors with M.E. Honorary Fellows Dr. William Weir and Dr. Nigel Speight co-author a newly published opinion piece that discusses the past, present and possible future of ME/CFS. In order to give context as to how ME/CFS came to be incorrectly viewed as a psychological disorder, the article draws on…Continue readingA Review of ME/CFS: Past, Present and Future