Doctors with M.E. proposes a simple solution to ensure success of the UK’s ME/CFS Delivery Plan. Following the then Secretary of State for Health and Social Care Sajid Javid’s announcement of a cross-nation delivery plan for ME/CFS, work has been underway at the Department of Health and Social Care to identify issues and find solutions.…Continue readingRights and Obligations in ME/CFS: Overcoming normalised disregard for standards
Tag: medico-legal
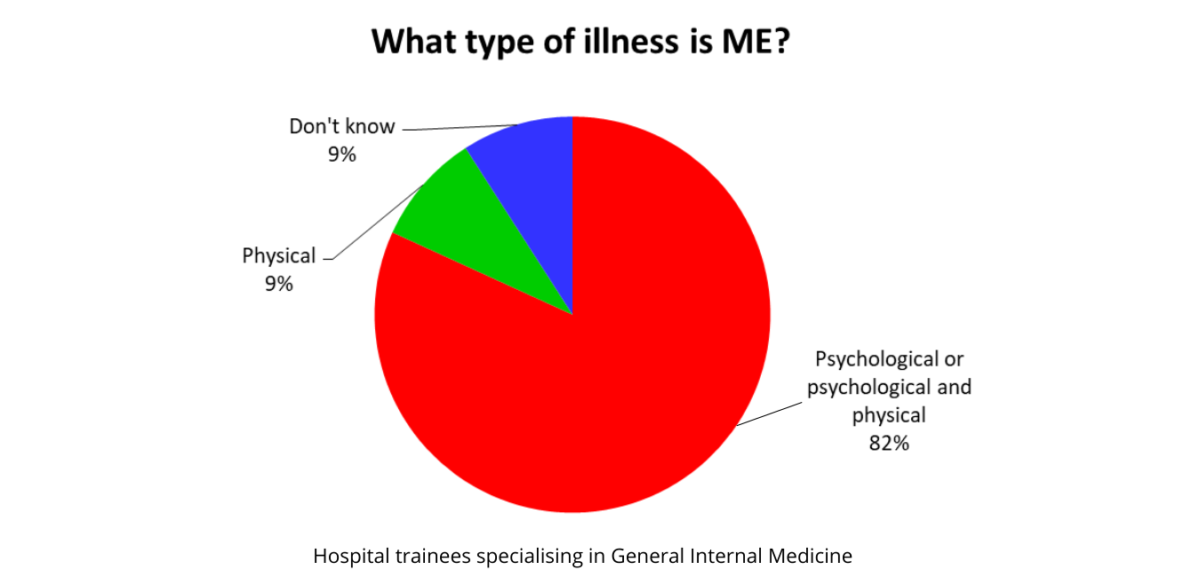
An Audit of UK Hospital Doctors’ Knowledge and Experience of Myalgic Encephalomyelitis Doctors with M.E. Founder and Director Dr. KN Hng, Director Dr. Keith Geraghty and Honorary Fellow Prof. Derek Pheby report their findings regarding ME/CFS knowledge and understanding amongst the medical community following an audit of hospital doctors at a training event in the…Continue readingPoor knowledge of ME/CFS among doctors puts patients at risk of harm
Doctors with M.E. stands ready to work with partners and organisations to bring medical education up-to-date and assist in developing the right services for ME/CFS patients. “The new guideline represents a positive and total paradigm change, uniting around the science, official disease classification and medico-legal compliance implications.” “This translation of scientific knowledge into clinical practice…Continue readingNICE 2021: A Triumph of Science over Discrimination
Doctors with M.E. Honorary Fellow Professor Ron Davis has issued a powerful statement condemning the failure of the UK National Institute of Clinical and Care Excellence to maintain its independence. He describes how it has instead accommodated vested interests, which perpetuate low standards and contra-scientific medical belief systems. “It is a travesty that NICE is…Continue readingDoctors with M.E. Honorary Fellow and founding father of the Human Genome Project comments on the NICE pause – “it is time for the UK to join the rest of the scientific community”
A call for change in the face of long-standing regulatory failure The following public communication from our Governance Board regards the opportunity for a new “Medical Regulatory Agency”, ready for the post-brexit and the machine learning digital eras. The letter also contains background pertaining to long-standing regulatory failure, unlawful medical norms, contra-scientific medical belief systems and…Continue readingPublic Letter to DHSC, MHRA, HoC, NICE: Call for a ‘Medical Regulatory Agency’
Today we announce the Doctors with M.E. Committees and Working Groups structures. These allow our Registrants to participate on ongoing or ad hoc bases, driven by their interests and events. Compliance, legal, policy and practice management professionals are fundamental to driving change. We have thus introduced Compliance and Policy Affiliate registration status, to ensure that all…Continue readingAnnouncing our Committees and Working Groups
We are very disappointed to hear of the ‘pause’ in publication of the NICE ME/CFS Guideline, already delayed in April. Following the hard work of the Guideline Development Group, we received news of the further delay of guideline publication with both dismay and profound concern for practitioners, their organisations and patients. Continued delay or deviation…Continue readingRapid Response and Expert Comment: NICE Guideline Delay and Accommodation of Unlawfulness
Care should be taken to reassess existing expertise sources in the field of post-viral disease and to distinguish between 1) the marketability or familiarity of expertise versus 2) disproportionate risk of divergence from legally sustainable standards. It is rare for institutionalised medical norms to exclude scientific consensus to the degree witnessed in this field. This…Continue readingExpertise procurement risk and reputational risk – medical and legal
Although the jurisdictional focus is the United Kingdom, direct equivalence can be found in other jurisdictions and markets. The policy, contractual and legal requirements on medical practice and indemnification provision follow a similar structure in most locales: neither official edict, professional body, private contract, the habitual nature of unlawful care nor eminently misinformed obfuscation can…Continue readingJurisdictions and markets (UK and international)
The absolute clarity of the legal status of ME/CFS is of immediate risk management importance, due its statutory, policy and third-party contractual implications frequently being ignored. These stem from habitually unlawful clinical judgement and arbitrarily discriminative administrative treatment that fail to account for the clarity of classification and associated lawful obligations. The absolute clarity of…Continue readingObligation to not negate ME/CFS legal status