Replacing Working Group Glass Ceilings with Enforceable Minimal Standards (clickable video chapters below and on YouTube site) Further to our recent statement regarding major UK announcements, the above presentation provides the solution to concerns about new DHSC ME/CFS Delivery Planning mechanisms and final implementation. Current glass ceilings will limit success of this new process and…Continue readingNormalised Medical Rule Breaking and the UK DHSC ME/CFS Review
Tag: Policy
Doctors with M.E Director and colleagues respond regarding the ignored importance of unpaid carers Doctors with M.E. Director, Dr. Nina Muirhead, has co-authored a BMJ Rapid Response regarding the topic of unpaid carers of people with ME. This important factor is largely ignored by the 2021 NICE ME/CFS Guidelines, the recent APPG report and the…Continue readingBMJ Rapid Response on Unpaid Carers
Doctors with M.E. warmly welcomes the UK Secretary of State for Health and Social Care’s recent inauguration of cross-nation delivery planning processes with his Chief Scientific Officer, Professor Lucy Chappell. Sajid Javid’s intervention on social media in the case of Maeve O’Neill was strikingly poignant, touching the hearts of many patients, carers and medics. We…Continue readingStatement on UK DHSC Announcements and the APPG Rethinking ME Report
Education, Collaboration and Policy Planning Initiative Following a generous private grant of resources targeted specifically at Project Florence, we are proud to publicly announce the initiative, which is now moving into a test phase following pandemic driven delays. Doctors with M.E. Registrants and Strategic Partners will benefit from a new digital platform that unifies education…Continue readingProject Florence Grant – Unified Digital Platform for Postviral Disease Professionals and Practitioners
Traducido por / Translated by Dr. Esther GarcésDoctors with M.E. Associate, Family Physician and RadiologistMédico especialista en Medicina Familiar y Comunitaria y Médico especialista en Radiodiagnóstico Contenido Descripción General La Encefalomielitis Miálgica (EM) —también conocida como Síndrome de Fatiga Crónica (SFC)— es una enfermedad orgánica crónica, compleja y multisistémica con consecuencias, a menudo, devastadoras. Se…Continue readingLa guía NICE EM/SFC aplicada a la práctica en atención primaria
Psychiatry and Psychology Working GroupDr Crowder BSc (hons) MB ChB FRANZCP, Associate, DwMEYochai Re’em, M.D., Associate, DwME | Website | Twitter What is ME/CFS? ME/CFS is a chronic, complex, multi-system biological illness with often devastating consequences. It affects all age groups including children, and all social classes. About 75% of sufferers are female. It has…Continue readingME/CFS: What Psychiatrists need to know
Section 1.16 of the NICE ME/CFS Guideline requires that health and social care providers ensure that all staff delivering care to people with ME/CFS have an understanding of the patient experience. Doctors with M.E. asked the CFS/ME Research Collaborative Patient Advisory Group (CMRC PAG) what patients make of the new NICE guideline. They shared the…Continue readingGetting compliant with NICE guideline – the patient experience requirement
Sumber asal diterbitkan dalam Bahasa Inggeris pada 15/1/2022. Diterjemahkan oleh / Translated byDr Hannah Nazri MBChB, BSc (Hons), MSc (Oxon)Doctors with M.E. Associate, clinician scientist, entrepreneur, and women’s rights & education advocate.Instagram | Twitter | LinkedIn | Blog Kandungan Gambaran keseluruhan Myalgic Encephalomyelitis (ME), juga dikenali sebagai Sindrom Kelesuan Kronik (Chronic Fatigue Syndrome – CFS),…Continue readingDari garis panduan kepada praktikal: Implikasi NICE ME/CFS buat doktor-doktor pakar perubatan keluarga (GP)
Publié initialement en anglais le 15/01/2022. Veuillez nous excuser, nous sommes conscients qu’il y a un problème de ponctuation. Nous faisons notre maximum pour le résoudre au plus vite. Traduit par Millions Missing France Contenu Vue d’ensemble L’encéphalomyélite myalgique (EM), également connue sous le nom de syndrome de fatigue chronique (SFC), est une maladie biologique…Continue readingMise en pratique : Ce que l’EM/SFC signifie pour les médecins généralistes, selon les préconisations du NICE
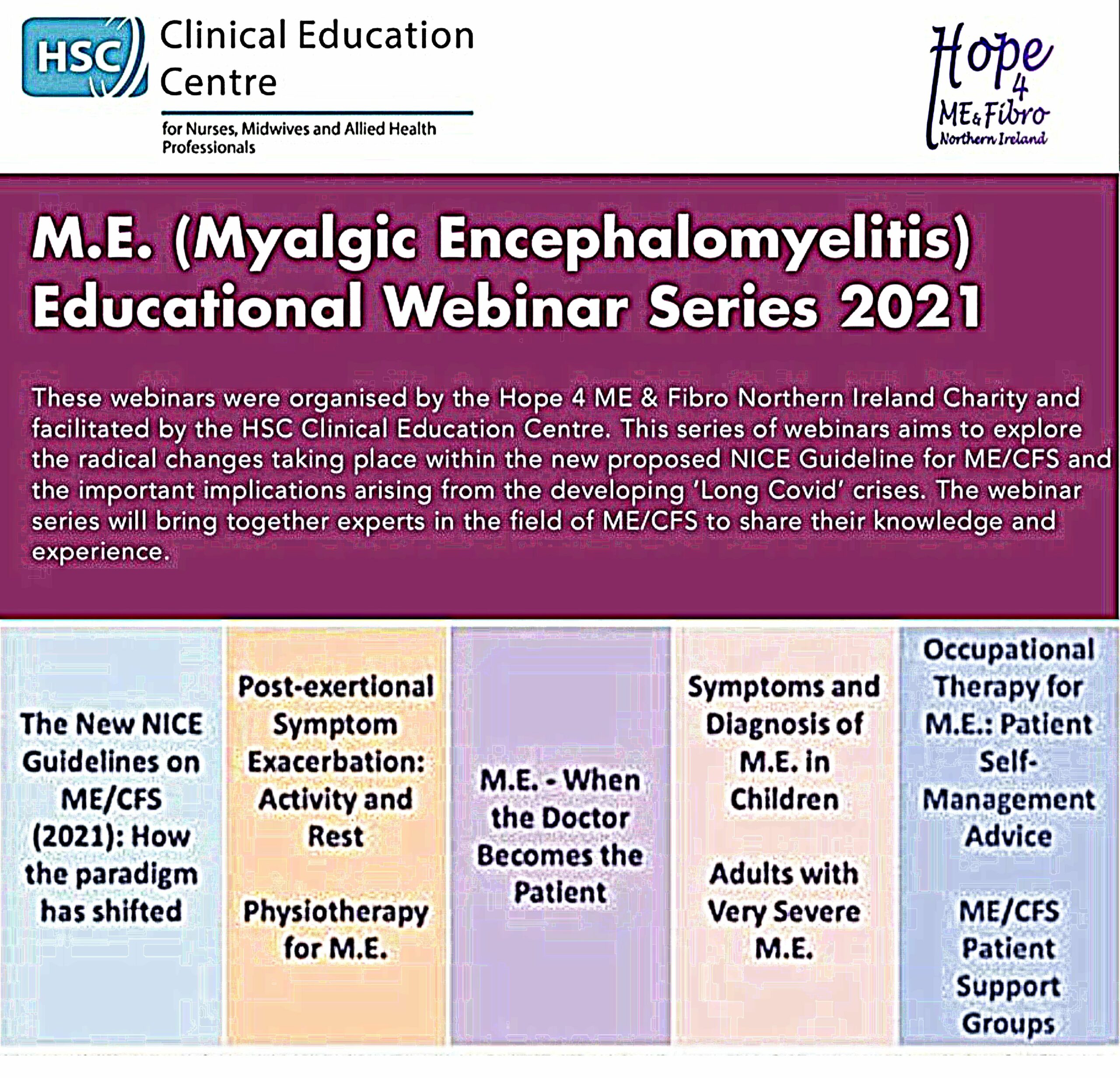
We are pleased to announce that the M.E. (Myalgic Encephalomyelitis) Educational Webinar Series organised by partner organisation Hope 4 ME & Fibro Northern Ireland and hosted by the Health and Social Care Clinical Education Centre of Northern Ireland is now available for public viewing. Featuring experts and speakers from Hope 4 ME & Fibro N.I.,…Continue readingMyalgic Encephalomyelitis: Northern Ireland Educational Webinar Series